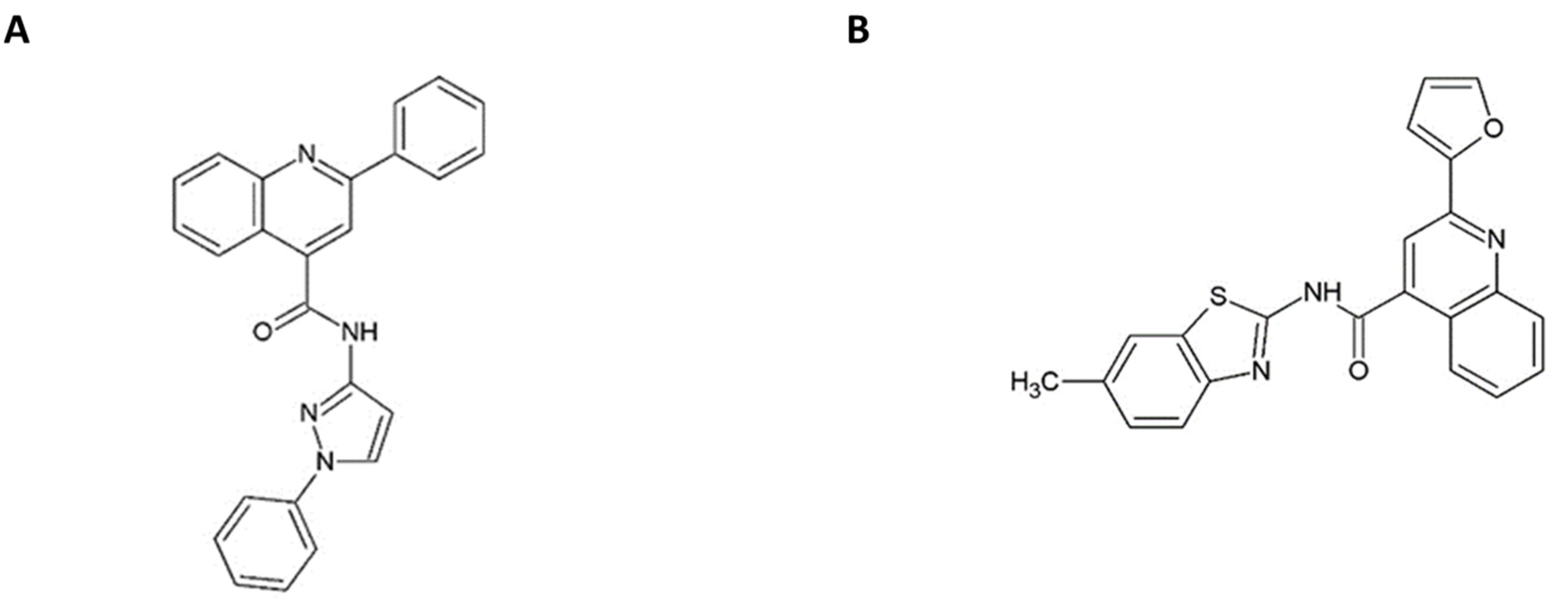
Molecules | Free Full-Text | Quinolinecarboxamides Inhibit the Replication of the Bovine Viral Diarrhea Virus by Targeting a Hot Spot for the Inhibition of Pestivirus Replication in the RNA-Dependent RNA Polymerase

Advanced Organic Chemistry: Reaction Mechanism, Strategy, Applications.... - Advanced Organic Chemistry: Reaction Mechanism, Strategy, Applications.
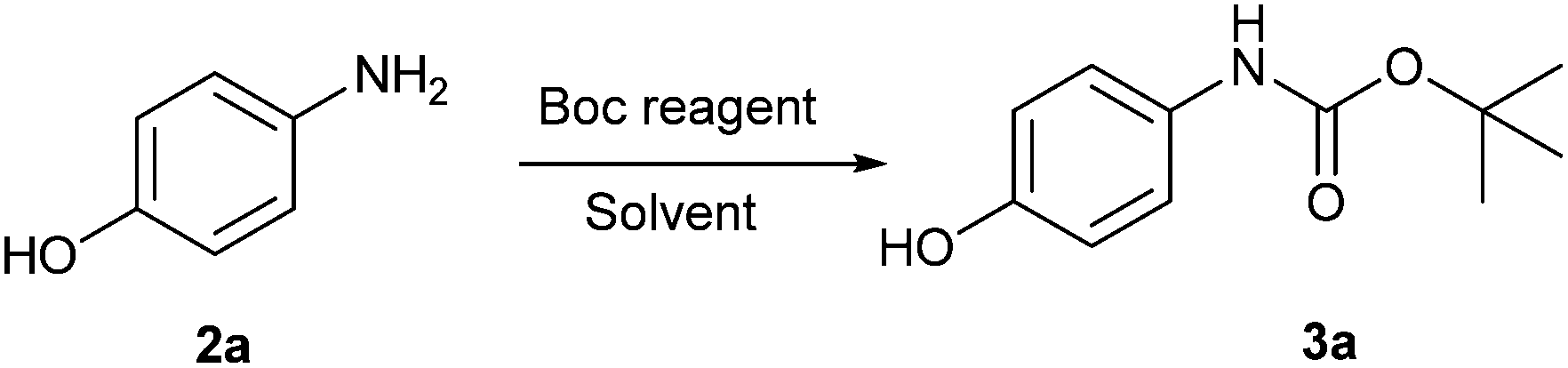
tert -Butyl(3-cyano-4,6-dimethylpyridin-2-yl)carbonate as a green and chemoselective N-tert -butoxycarbonylation reagent - New Journal of Chemistry (RSC Publishing) DOI:10.1039/C9NJ00885C
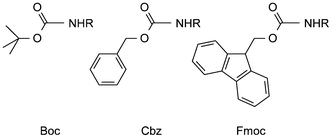
Dual protection of amino functions involving Boc - RSC Advances (RSC Publishing) DOI:10.1039/C3RA42956C
![PDF) Imidazole and Trifluoroethanol as Efficient and Mild Reagents for Destruction of Excess Di-tert-butyl Dicarbonate [(BOC)2O] PDF) Imidazole and Trifluoroethanol as Efficient and Mild Reagents for Destruction of Excess Di-tert-butyl Dicarbonate [(BOC)2O]](https://www.researchgate.net/profile/Yochai-Basel/publication/244567326/figure/fig1/AS:500353444651009@1496304965807/Imidazole-and-Trifluoroethanol-as-Efficient-and-Mild-Reagents-for-Destruction-of-Excess_Q320.jpg)
PDF) Imidazole and Trifluoroethanol as Efficient and Mild Reagents for Destruction of Excess Di-tert-butyl Dicarbonate [(BOC)2O]

PDF) p-Nitrobenzyloxycarbonyl (pNZ) as an Alternative to Fmoc for the Protection of Amines in Solid-Phase Peptide Synthesis
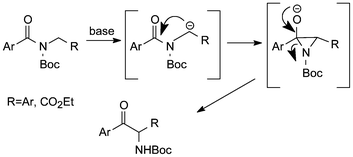
Dual protection of amino functions involving Boc - RSC Advances (RSC Publishing) DOI:10.1039/C3RA42956C
![Isocyanide based [4+1] cycloaddition reactions: an indispensable tool in multi-component reactions (MCRs) - Chemical Communications (RSC Publishing) DOI:10.1039/C6CC01562J Isocyanide based [4+1] cycloaddition reactions: an indispensable tool in multi-component reactions (MCRs) - Chemical Communications (RSC Publishing) DOI:10.1039/C6CC01562J](https://pubs.rsc.org/image/article/2016/CC/c6cc01562j/c6cc01562j-s4_hi-res.gif)
Isocyanide based [4+1] cycloaddition reactions: an indispensable tool in multi-component reactions (MCRs) - Chemical Communications (RSC Publishing) DOI:10.1039/C6CC01562J













