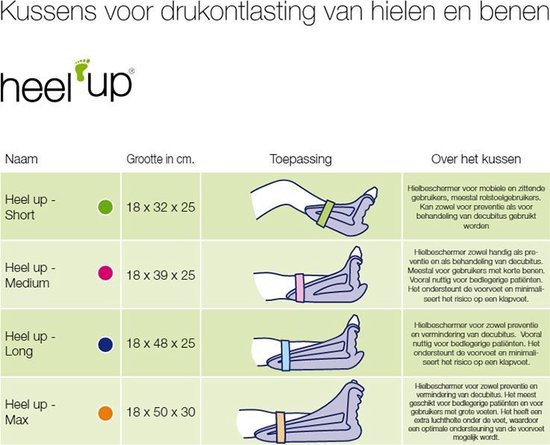
EN ISO 10993-18:2020 - Biological evaluation of medical devices - Part 18: Chemical characterization

SIST EN ISO 10993-1:2021 - Biological evaluation of medical devices - Part 1: Evaluation and testing

SIST EN ISO 10993-1:2021 - Biological evaluation of medical devices - Part 1: Evaluation and testing